Quick Contact

MHRA Registration
MHRA Registration for medical device and in vitro diagnostic device is mandatory before being placed on the UK market from January 1, 2021. The registration of devices, on the other hand, is staggered according to the risk class of the devices and the GMDN Code’s.
MHRA Registration must be renewed one year after your registration application or confirmation was made and every two years after this date. Failure to renew your registration will result in the removal of your records from our database, after which you will need to complete a new registration application or you will no longer be able to place your device on the UK market.
MHRA Registration Timeline
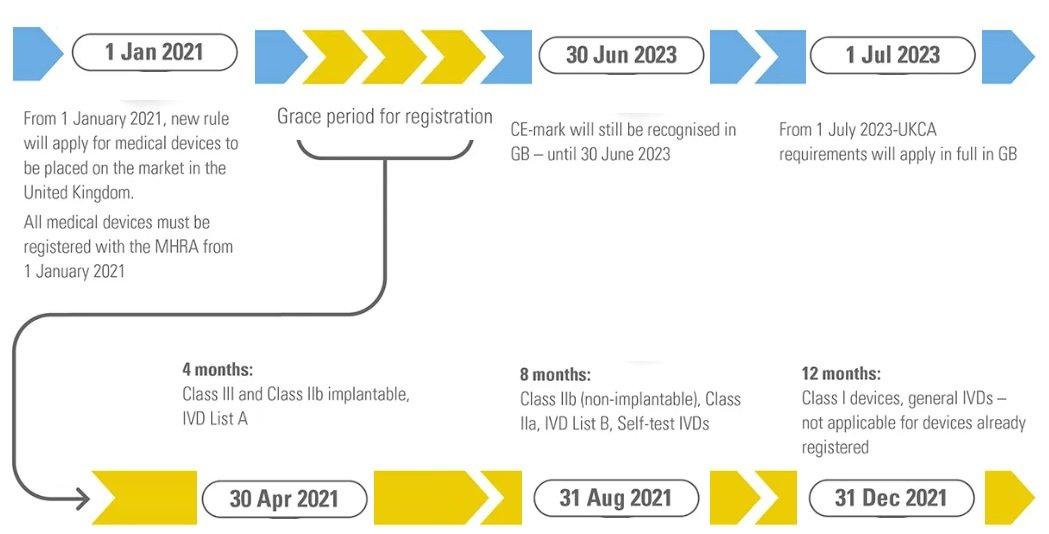
All medical device and in vitro diagnostic device must be registered with the MHRA before being placed on the UK market on January 1, 2021. The registration of medical devices, on the other hand, is staggered according to the risk class of the devices and the GMDN Code’s
To allow free trade without interception, pre-existing CE-marked devices are allowed until December 31, 2021. However, new products placed on the UK market for the first time after January 1, 2021, should carry a valid UKCA Marking.
The difference between CE Marking and UKCA certification is administrative. It reflects that UKCA requires information in English, and UK-approved bodies are authorized to issue UKCA certificates compared to Notified Bodies earlier. The product scope, technical requirements (essential requirements, standards), and conformity assessment procedures will all be alike.
Do you need an email containing full details about MHRA within 2 minutes?
MHRA Registration Importance
You must obtain MHRA Registration of Medical Devices and IVDs to sell, lease, rent, or exchange your product on the UK market. Make arrangements to register with the Medicines and Healthcare Products Regulatory Agency (MHRA). The UK MDR 2002 requires you to inform the MHRA before placing your device on the market in the United Kingdom. All non-UK businesses must designate a UK Responsible Person with a registered place of business in the UK to register with the MHRA.
The following devices must be registered with the MHRA before 1 May 2021.
- » Active implantable medical devices
- » Class III medical devices
- » Class IIb implantable medical devices
- » IVD List A products
The following devices must be registered with the MHRA before 1 September 2021.
- » Class IIb non-implantable medical devices
- » Class IIa medical devices
- » IVD List B products
- » Self-test IVDs
The following devices must be registered with the MHRA before 1 January 2022.
- » Class I medical devices
- » General IVDs
MHRA Registration Search
Documents for MHRA registration
A. Manufacturer (Facility) Information:
- » Legal manufacturer name, address, designation, telephone and email
- » Activity carried out by the legal manufacturer.
- » Person responsible to communicate with MHRA (Name, Email & Contact Information)
- » Mutually signed agreement with legal manufacturer and UK Responsible person.
B. Medical Device Information:
- » Basic UDI-DI ( Presently not enforced, but soon will come into effect)
- » Medical device brand/trade or proprietary name
- » Device model or versions
- » IFU / User Manual / Catalogue reference number
- » UK Approved Body Or Notified Body name and address for all classes other than class I
- » Type of Sterilization (If applicable)
- » Details about Latex and phthalates.
- » Medical Device MRI compatibility issues
- » Conformity assessment certificates
- » Signed and dated Declaration of Conformity
- » Technical File latest revision number with date.
We act as UK responsible person for all foreign medical device manufacturing wishing to sell in UK. We registration all manufactures with MHRA and list the devices. Contact us for your immediate requirements.
Frequently Asked Questions
Do I3CGlobal help with Technical Documentation?
Yes, we are medical device regulatory consultants and UKRP service providers. We help manufacturers submit technical documentation to Certification bodies for UKCA
Is I3CGlobal accredited with MHRA?
UKRP service providers are not accredited with MHRA. We are registered as a business establishment in the UK. We are providing service for many foreign service providers.
UKRP and MHRA Registration timeline
UKRP agreement preparation and mutual signing will be done in 3-4 working days and MHRA registration may take around 15 working days usually.
Placing goods in Northern Ireland
Manufacturers in the United Kingdom will need to designate a European Authorized Representative to place goods in the EU or Northern Ireland. CE Certification will be needed in Northern Ireland, and EU MDR and EU IVDR are applicable.
Devices with a CE marking can still be sold in the UK?
Devices with a CE marking can still be sold in the UK until June 30, 2023, but from July 1, all medical devices and in vitro diagnostics will require a UKCA certification to be sold in the United Kingdom.