ISO Sampling Plan Standard
- ISO sampling plan criteria are lot sizes, inspection levels, acceptable quality levels, sample size code letters, and acceptance and rejection points.
- Three types of ISO sampling plan, single, double, and multiple.
- Three types of Inspection levels: Normal, reduced, and Tightened inspections.
- The AQL and the sample size code letter shall be used to obtain the sampling plan.
Get in-depth insights into ISO 13485 certification, the global standard for medical device quality management. Learn its requirements, benefits, and compliance process.
We provide ISO sampling plan guidance as per the EU MDR & IVDR CE Marking, US FDA 510k and ISO 13485 Certification. Contact us for more information or fill out the form for a detailed quote.
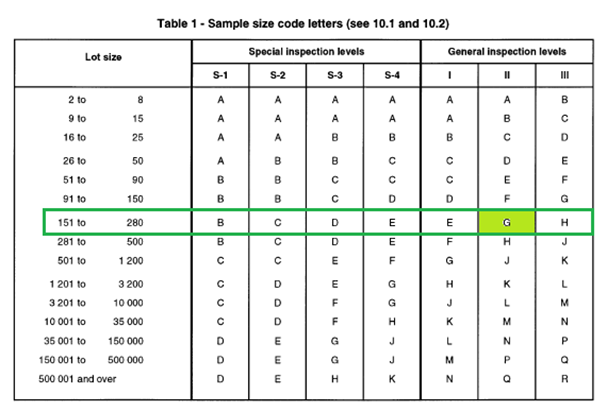
The selection of sample size is explained
Step 1: Decide the lot size and the inspection level.
Step 2: Assume a lot size of 151- 280 and inspection level G- II (general inspection II), then the inspection level code is obtained as “G” from the below table 1. In short, the lot size and the inspection level allow us to select the sample size code letter.
Step 3: Select the type of ISO sampling plan: single / double / multiple sampling. The acceptability of the lot is based on the percent of nonconforming inspection.
Step 4: Select the Inspection level- Normal/ reduced / Tightened inspection.
Step 5: The AQL and the sample size code letter shall be used to obtain the ISO sampling plan.
Step 6: If consider AQL as 4, and the code for a lot size of 151- 280 is G (table1) and single ISO sampling plan with normal inspection level. Then the required sample size will be 32 units with the Acceptance number and Rejection numbers are 3 and 4 respectively (Table 2.A).

*The tables are from ISO 2859-1
ISO 2859 Sampling Plan International Standards
- ISO 2859-1: ISO Sampling procedures for inspection by attributes – Part 1: Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot inspection.
- ANSI/ ASQ Z1.4 & Z1.9: Sampling Procedures and Tables for Inspection by Attributes.