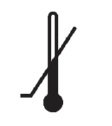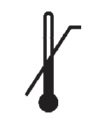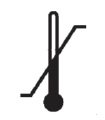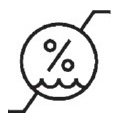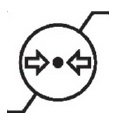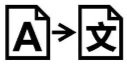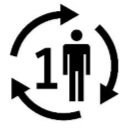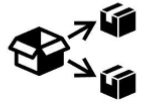
Medical Device Symbols
Medical Device Symbols are to be used with medical device labels when supplied. These symbols are a part of the regulatory requirements by leading regulatory bodies including the EU and US FDA. Its provide essential information about the device, such as manufacturing date, expiry date, warnings and precautions.
Medical device symbols use standardized icons that are internationally recognized. This gives healthcare professionals, patients, and caregivers a quick and clear way to convey important information, even across language barriers. They are often accompanied by text in the user manual or leaflet.
Looking for additional information about MDR CE Marking, we will send you details in 2 minutes over email.
Applicable Harmonised Standards
- EN 1041:2008: Information supplied by the manufacturer of medical devices.
- EN 15986:2011: Requirements for labeling of medical devices containing phthalates.
- EN ISO 3826-2:2008: Plastics collapsible containers for human blood and blood components – Part 2: Graphical symbols for use on labels and instruction leaflets.
- EN ISO 15223-1:2012: Medical devices Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements.
In-Vitro Diagnostic and Medical Device Symbols
Disclaimer
The above Medical Device Symbols images are not in the required pixels or size and are not the exact representation of the standard. The above information is intended for informational purposes only and should not be construed as legal advice for regulatory submissions.
How to build a Technical Documentation as per MDR 2017/745? We have the answer and the right team to do it for you. Know more about medical device technical file.
Frequently Asked Questions
Where can we purchase the symbols?
Is it mandatory all symbols must be in the primary label?
No. If you have space constraints, you may use a single logo “read information for use” in the primary label. The remaining symbols are fixed in the IFU/User manual.