IVDR Regulation
The new European Union IVDR regulation described in Article 10(4), Technical File, is a mandatory requirement for all manufacturers applying for IVDR CE certification with any notified body. The IVDR replaced the IVDD and entered into force on May 26, 2017, with an implementation due date of May 26, 2022. All new in vitro diagnostic devices placed on the EU market, including class A non-sterile devices, must follow the new IVDR regulation. The major changes are as below:
› IVDR classification based on Rule
› Requirements for clinical evidence and post-market performance follow-up
› Increased traceability of devices (UDI)
› Person Responsible for regulatory compliance
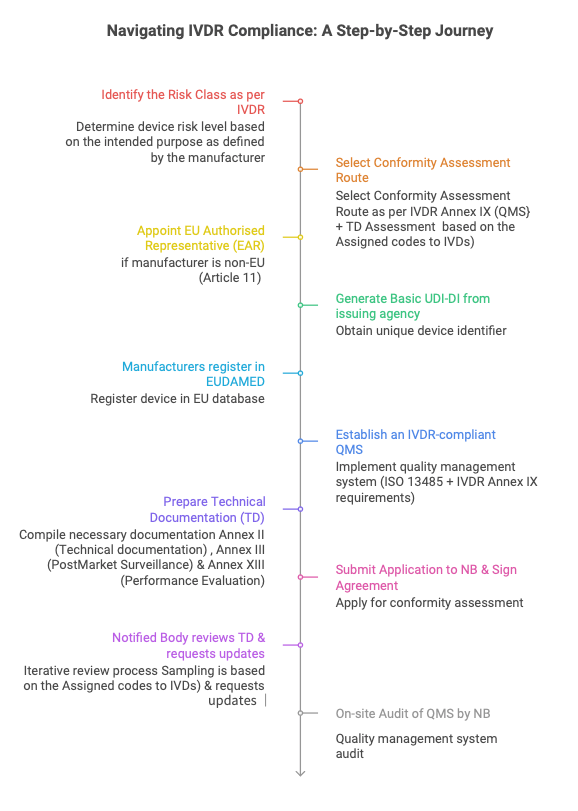
IVDR Regulation 2017/746 Implementation Strategy
> Device identification and planning begin with a GAP analysis, followed by the formation of a project management team that is responsible for coordinating all activities with consultants and the Notified Body
> Start technical documentation by conducting a performance evaluation and developing a comprehensive report. Transfer the relevant data from the Performance Evaluation Report (PER) to the Technical Documentation file. The facility should be thoroughly assessed by consultants to ensure strict implementation of the Quality Management System (QMS). Consult subject matter experts on study design and formulation to avoid any potential impact on device specificity and sensitivity
> Notified body conformity assessment: Submit technical documentation to the notified body, respond to file review comments,
> Performance evaluation must be designed to be a continuous activity, involving the gathering of clinical evidence throughout the lifespan of the In-vitro diagnostic device. This is achieved with the assistance of the post market performance follow up process. No European manufacturers must involve the service of EU Representative.
Request for an email with full details within minutes? Privacy Policy>>
An IVD Device confirmation Under the New IVDR Regulation
According to EU 2017/746 IVDR Regulation, an in vitro diagnostic (IVD) medical device is any medical device that consists of a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment, software, or system. Whether used alone or in combination, it is intended by the manufacturer for in vitro examination of specimens—such as blood and tissue donations—derived from the human body, primarily to provide information on one or more of the following:
› A physiological or pathological process or state
› Congenital physical or mental impairments
› A predisposition to a medical condition or disease
› The safety and compatibility of potential recipients
› Predicted treatment responses or reactions
› The definition or monitoring of therapeutic measures
Team I3CGLOBAL provides strategic guidance for IVDR Regulation. We have the necessary expertise and know-how, manpower to navigate EU 2017/746 regulations for small, medium, and large-scale manufacturers globally by supporting all stages of technical documentation. Outsource the work to I3CGLOBAL and rest assured with us!
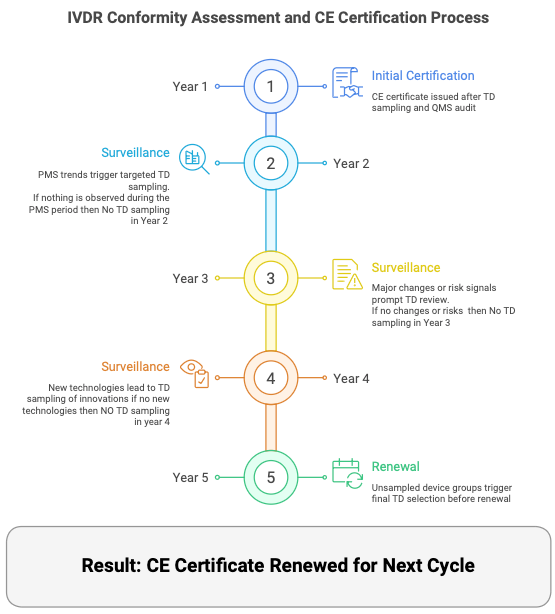
IVDR Transition Timeline
In January 2024, the European Commission released a proposal aiming to modify the IVDR 2017/746, thereby prolonging the transition periods. The primary rationale cited for this action is the shortage of notified bodies. While this doesn’t alter the IVDR’s application date, set to remain on May 26, 2022, the extended deadlines afford manufacturers and notified bodies additional time to navigate the IVDR conformity assessment process. Consequently, this ensures that safe and efficient IVD products aren’t needlessly discarded.

Extended Transition Deadlines for IVD Devices
The IVDR Regulation introduced significant developments in the regulatory structure of in vitro diagnostics in the European Union. Acknowledging these alterations, the European Commission extended the deadlines for compliance.
The In Vitro Diagnostic Directive has been replaced by the IVD Regulation EU Regulation 2017/746 and becomes effective May 26, 2022. An amendment on behalf of the European Commission was prepared to extend the transition periods for life-saving IVDs so that they can stay on the market longer while giving producers more time to take care of the new, more complex requirements.
Such an extension was warranted by the incompleteness of preparations by the stakeholders involved: manufacturers notified bodies, and patients. Further extensions in the deadlines for the transition of IVDs under the IVDR have been set out in Regulation (EU) 2024/1860, published on July 9, 2024.
A key change brought about by Regulation (EU) 2023/607 also removed the ‘sell-off provision’. This means that IVDs already placed on the market can continue to be made available or put into service until the revised expiry of the certificate or until the shelf life of the device.
Latest Major Updates from EU: The extended timelines are based on the risk class of the IVD and whether the device was previously certified under the IVDD or is self-declared. Below are the updated deadlines:
Legacy Devices: Applies to devices with an IVDD certificate (Annex II List A, List B, and self-tests) issued between May 25, 2017, and May 25, 2022., and has not been withdrawn which requires a notified body under IVDR. The following flow determines the eligibility for extension and market placement:
Scenario 1 : For IVDs with a valid certificate under the IVDD.
Conditions for Extension: To extend the certificate’s validity and continue placing the device on the market:
- The manufacturer must lodge an application (before May 26, 2025) and establish a written agreement with a notified body (before September 26, 2025) for the device or substitute device before the certificate expires.
- The Quality Management System (QMS) must comply with IVDR requirements by May 26, 2025.
Final Deadline: If the above conditions are met, the device can be placed on the market or put into service until December 31, 2027.
Scenario 2 : IVDs with an expired certificate before 9 July 2024
The same transitional periods as for IVDs with a valid certificate have been granted, provided one of the below conditions are fulfilled:
- An application submission or written agreement with a notified body is in place before the certificate expiry date.
- The competent authority has granted a derogation under IVDR Article 54(1).
- The competent authority requires the manufacturer to perform the applicable conformity assessment procedure under Article 92(1).
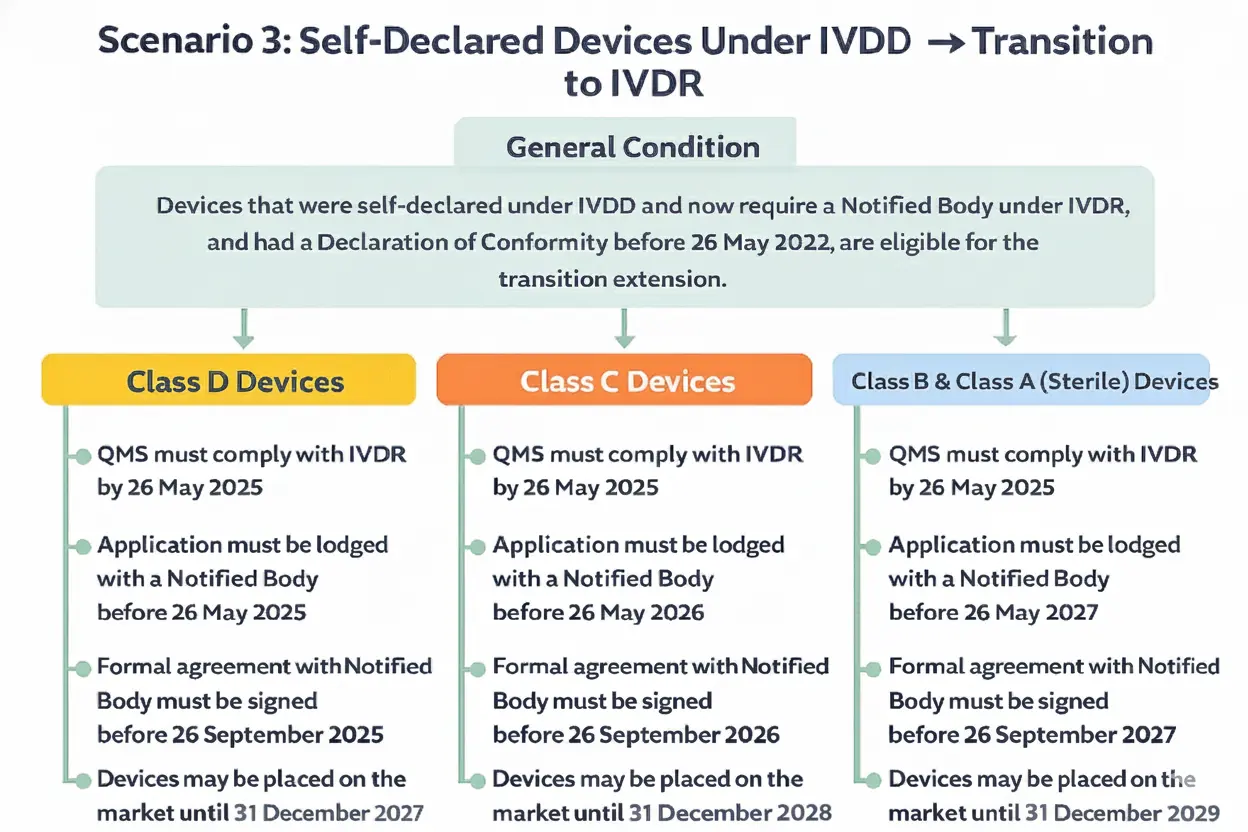
Scenario 3 : Self Declared Devices Under IVDD
For devices that were self-declared under the IVDD require a notified body under IVDR and had a declaration of conformity before May 26, 2022, the extension also applies.
Class D Devices
- The Quality Management System (QMS) must comply with IVDR by May 26, 2025.
- An Application must be lodged with a notified body before May 26, 2025.
- A formal agreement with the notified body must be signed before September 26, 2025.
Devices can be placed on the market or put into service until December 31, 2027.
Class C Devices
- The Quality Management System (QMS) must comply with IVDR by May 26, 2025.
- An Application must be lodged with a notified body before May 26, 2026.
- A formal agreement with the notified body must be signed before September 26, 2026.
Devices can be placed on the market or put into service until December 31, 2028.
Class B and Class A (Sterile) Devices
- The Quality Management System (QMS) must comply with IVDR by May 26, 2025.
- An Application must be lodged with a notified body before May 26, 2027.
- A formal agreement with the notified body must be signed before September 26, 2027.
Devices can be placed on the market or put into service until December 31, 2029.
No transitional period applies to class A devices (except for class A sterile devices), because they do not require the involvement of a notified body in the conformity assessment. New devices introduced to the market after May 26, 2022, must fully comply with the IVDR requirements. No transition period applies to these devices.
Frequently Asked Questions
What are the Conditions for Using Extended Timelines?
- Devices must still comply with IVDD requirements during the transition period.
- No significant changes in design or intended purpose are allowed.
- The device must not pose an unacceptable risk to health or safety.
- Post-market surveillance, vigilance, and registration must comply to IVDR standards.
What are the Impacts of Extended Timelines on IVD Manufacturers?
The extended timelines provide much-needed relief to manufacturers, allowing them to:
- Conduct a thorough gap analysis and implement the necessary changes to meet IVDR requirements.
- Secure notified body capacity, which has been a significant bottleneck in the transition process.
- Ensure uninterrupted availability of essential IVDs in the EU market.
What requirements of IVDR regulation manufacturers have to meet during the extension period?
- Post-market surveillance (PMS)
- Vigilance
- EUDAMED
- EN ISO 13485:2016
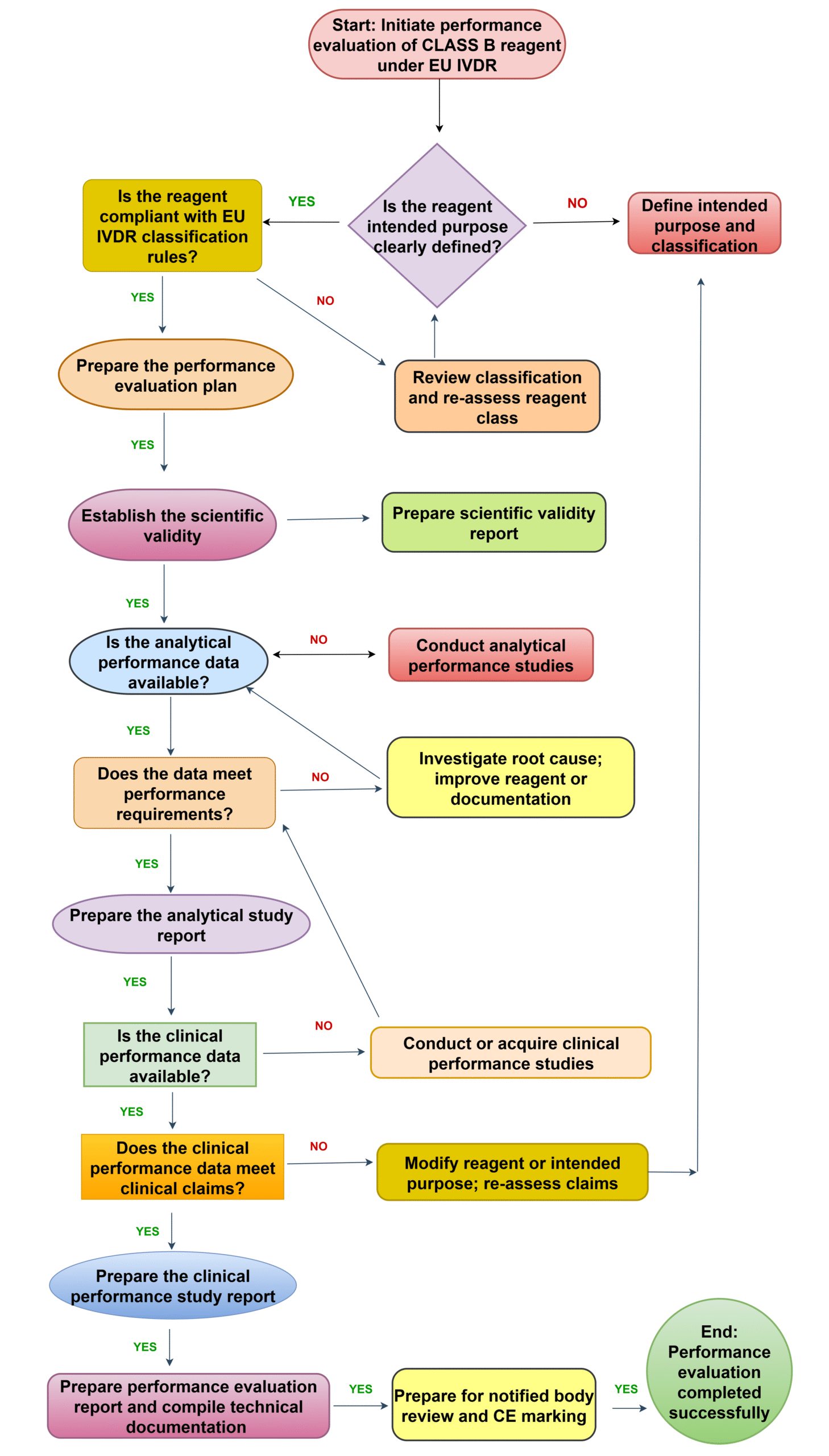
How important performance evaluation in IVDR Regulation?
The IVDR performance evaluation involves a combination of Scientific validity, Analytical performance, Clinical performance and Scientific peer-reviewed literature. The PER contains all the clinical evidence including the scientific validity report, analytical performance report, and clinical performance report. 2017/746 IVDR regulation more focus on PER.