Quick Contact
Just supply the infomation requested by us. We develop your Technical Documentation for you

Class III Medical Device
According to the New Medical Device Regulation (MDR 2017/745), Class III Medical Devices are considered to pose the highest risk class. Medical Devices constitute high-risk devices such as Pacemakers, Brain spatula, Copper-T IUDs, Cardiovascular sutures, etc. Permanent monitoring is required throughout its lifetime.
For Class III Medical Device CE Certification the declaration of conformity is backed up by notified body assessment followed by expert panel consultation and sometimes clinical evaluation consultation based on the type of device is inevitable.
Do you need an email containing full details within 2 minutes?
Class III Medical Device Conformity Assessment Route (Implantable Device)
For high risk implantable Class III Medical Device (including devices with medicinal substances, human tissue or animal tissue derivatives with TSE risk, Class III Rule 21 devices), the CE marking conformity assessment route is as follows:
- Consultation (2001/83/EC, EC/726/2004, 2004/23/EC, EU/722/2012)
- Clinical evaluation consultation Procedure as per Annex IX sec. 5/Annex X sec. 6
- Declaration of conformity as per Annex IV
- Affixing the CE Mark as per Annex V CE marking
Class 3 Medical Device Conformity Assessment Route (Non-Implantable Device)
For Non-Implantable Class 3 medical device (including devices with medicinal substances, human tissue or animal tissue derivatives with TSE risk, Class 3 Rule 21 devices), the CE mark conformity assessment route is as follows:
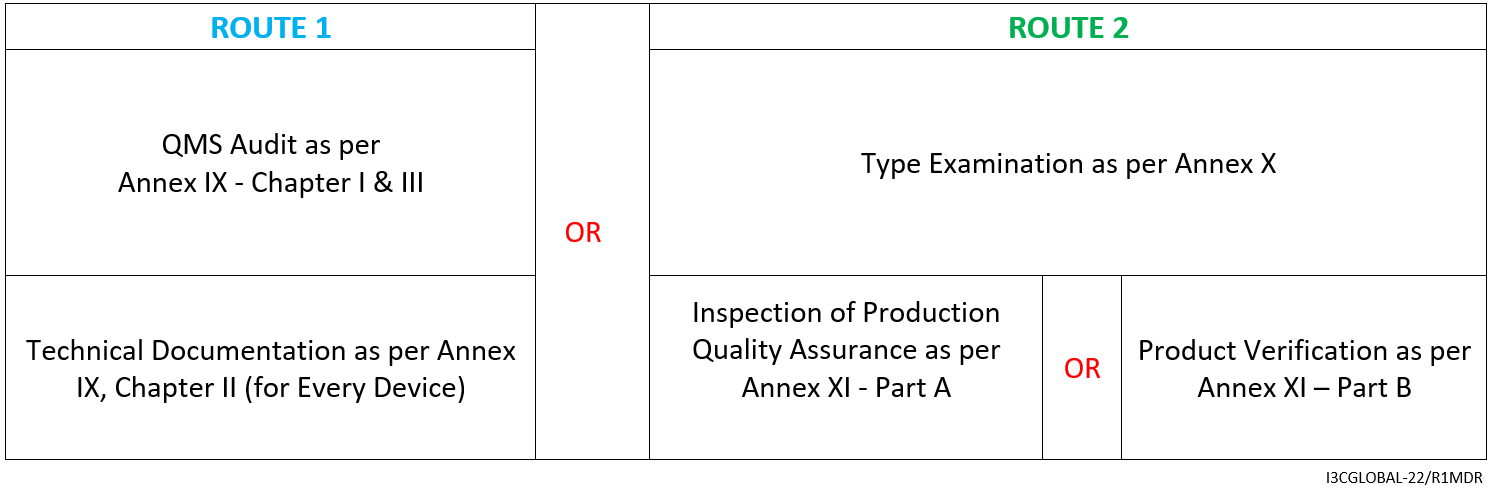
- Consultation (2001/83/EC, EC/726/2004, 2004/23/EC, EU/722/2012)
- Declaration of Conformity as per Annex IV
- Affixing the CE mark as per Annex V CE marking
Are you planning to obtain Class III medical device CE Certification through a Notified Body? Rest assured, we are experts and provide services globally by MDR 2017, ensuring full compliance with all Notified Body requirements.
Class III Medical Device Consultants
It is compulsory to have experts on board who have previous experience with Class III medical device, EU compliance and Clinical Evaluation for a successful outcome! Roles of I3CGLOBAL are as follows:
- Technical Documentation guidance & preparation
- Notified Body submission and answering to the review comments
- Guides in Biological Evaluation
- Review the external protocols and reports
- Prepares Clinical Evaluation Report as per Meddev 2.7/1 Rev 4
- Conducts Risk Analysis and prepares Risk Management File as per EN ISO 14971
- Arrange Notified Body and coordinate with them till the issue of the CE Certificate
- Arranges EU Representative from the European Union
- Arrange a Free Sale Certificate from the European Union
- Develops Post Market Surveillance plan, Post Market Clinical follow-up plan and report and Periodic Safety Update Report.
Frequently Asked Questions
Does Clinical investigations and equivalence shall be performed for Class III Medical Device CE Marking?
A. If equivalent devices have sufficient clinical data and the manufacturer conducted PMCF studies
- Certain design modifications of the manufacturer’s device
- Equivalent devices marketed by other manufacturers, where the contract allows ongoing access to data
- Existing CE-marked devices
B. If the devices have sufficient clinical data compliant with CS if available
- Sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors
What are the devices covered in the new device reclassifications as per Annex VIII?
- Implantable contraceptives
- Absorbable non-implants (eg skin or GI)
- Total and partial joint replacement implants
- Implants in contact with the spinal column
- Devices incorporating nanomaterials
- Non-invasive devices used in direct contact with human cells for IVF
- Devices incorporating human-derived substances
- Software that could have an impact that may cause death or irreversible deterioration of a person’s state of health

