Quick Contact

Class 1s Medical Device
The sterile medical devices are simple device with low risk manufactured and packed by the manufacturer in sterile condition. Class 1s medical device is normally used without a connection to an active device. The user must ensure device sterility before use by checking for any damage in the packaging and discarded after use.
Are you planning to apply MDR CE Certification for Class 1s medical device? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Class 1s Medical Device Technical Documentation
Below image explains the process and important documentation requirements for CE Certification for (class 1s) sterile low risk single use medical devices.
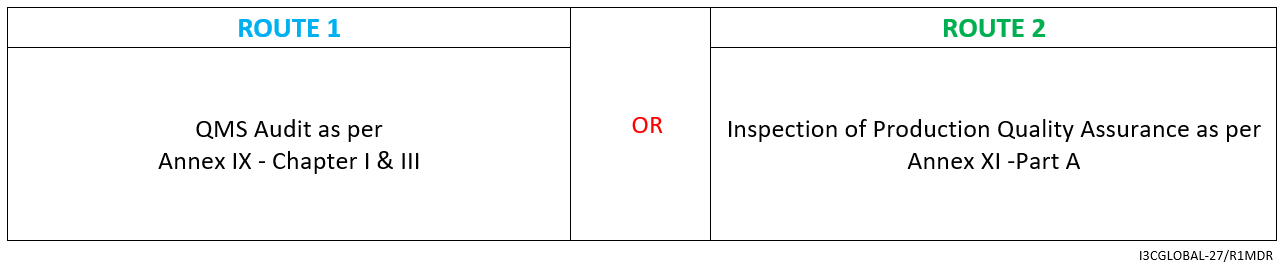
Class 1s Medical Device CE Conformity Assessment Route
MDR class 1s sterile medical device conformity assessment route for CE certification is detailed below.
Do you need an email containing full details within 2 minutes?
MDR specific requirements for Class 1s medical devices
For Class 1s medical device that are intended to be sterile, the MDR sets forth specific requirements:
- The medical device must be sterilized using a validated process. The method of sterilization should be appropriate for the device’s material and design. Common methods include steam, ethylene oxide, or radiation. The technical documentation should cover detailed sterilization protocol covering IQ,OQ and PQ. The Validation report must have a conclusion about the final output of validation and all supporting data to establish validation conduct.
- Manufacturers need to maintain detailed records of the sterilisation process, including validation and routine control procedures. This documentation is part of the technical documentation required for the device and should be available for review by regulatory authorities if needed.
- The sterilization process should comply with relevant standards, such as ISO 11135, ISO 11137, ISO 17665 and ISO 17664 , to ensure that the device is consistently sterile.
- Sterile Class 1s medical device must be packaged in a way that maintains their sterility until the point of use and throughout the shellfire period. The packaging should be sealed and labeled to ensure that it is not compromised. Sealing validation protocol, report and support data must be attached to the technical documentation for early notified body approval.
- The medical device must be labeled with appropriate information indicating that it is sterile. The label should also include details about the sterilization method used, and any instructions for use or handling to maintain sterility.
Role of I3CGLOBAL Consultants in Class 1s medical device CE Certification?
I3CGLOBAL Consultants play a significant role in assisting low risk class 1 sterile medical device manufacturers with the CE certification process. These devices, under the EU MDR, require indepth technical documentation. We help manufacturers develop a regulatory strategy that aligns with the specific requirements for sterile devices under the MDR by identifying applicable regulations, guidance documents, and standards.
- We consultants prepare the technical documentation file ensuring it meets MDR requirements and submit to NB for review.
- Ensure proper clinical evaluation plan, literature search and Clinical Evaluation Report (CER) complying with MDR Article 61 and Meddev 2.7/1 rev 4 guidelines.
- Review, guide on critical topics such as sterilisation and sterile pouch sealing and its validation studies.
- Review and support in the risk analysis process and benefit risk estimation as per ISO 14971
- Guide the manufacturer through the performance and functional testing.
- Liaison between the manufacturer and the Notified Body, during technical file review process.
I3CGLOBAL Consultants provide comprehensive support to reusable surgical instruments manufacturers ensuring complete compliance and documentation for CE Certification.
Are you planning to apply MDR CE Certification for Class 1s medical devices? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Frequently Asked Questions
How long the class 1s medical device CE certification process?
Generally the process is divided into 3. They are explained below
- Technical documentation file preparation usually with the help of consultants may take around 4 months.
- Notified Body class 1s medical device technical file review and approval by a notified body. Usually this process will take around 8 months.
- Onsite audit by Notified Body team and issue of CE Certificate takes approximately 2 months.
So the total timeline is expected around 12-14 months.
How does ethylene oxide sterilization work?
The ETO sterilisation process involves exposing the class 1 medical device to ethylene oxide gas in a controlled environment. The etheline gas penetrates the device and its packaging, killing microorganisms, thereby sterilising the device. The process typically occurs in a sterilisation chamber where temperature, humidity, and gas concentration are correctly controlled.
What are the benefits of using ethylene oxide sterilization?
- EtO can penetrate porous materials and complex geometries, making it suitable for intricate devices.
- It is ideal for heat-sensitive materials and devices that cannot endure high-temperature steam sterilisation.
- EtO is effective against a wide range of microorganisms, including bacteria, viruses, and fungi.
What are the disadvantages of ethylene oxide sterilisation?
- The entire sterilisation and aeration process can be time-consuming.
- Ethylene oxide is hazardous, requiring stringent safety measures for handling and disposal.
- Residual ethylene oxide must be properly aerated to ensure safety before the device is used.
What is radiation sterilization?
Radiation sterilisation uses ionising radiation to kill microorganisms on medical devices. The most common types of radiation used are gamma rays, electron beams (e-beams), and X-rays. The radiation damages the DNA of microorganisms, rendering them unable to reproduce and thus effectively sterilising the device.
What are the benefits of radiation sterilization?
- Effective for devices with complex nature and packaging.
- The process can be completed relatively quickly compared to ETO and other methods.
- Suitable for a wide range of materials and products, including those sensitive to heat.
- Accepted by all leading regulatory bodies
What are the disadvantages of radiation sterilization?
-
- Prolonged exposure to high doses of radiation can degrade certain materials, affecting their strength and integrity.
- Initial setup costs for radiation facilities and equipment can be high.
- Sterilization process validation are expensive
- Different radiation types have varying penetration abilities, which may limit their use depending on the device’s packaging and density.
- Highly restricted the use and availability of raw materials. Separate laws are applicable for the construction of test facilities.
