Quick Contact

Class 1r Reusable Surgical Instruments
The class 1r reusable surgical instruments are intended for surgical use in cutting, drilling, sawing, scratching, scraping, clamping, retracting, clipping or similar procedures, without a connection to an active device. The user must ensure device sterility before use by cleaning and sterilizing it after every use as per the manufacturer’s instruction.
All manufacturers previously sold in Europe under MDD self-declaration must obtain an MDR CE certificate under Annex IX Chapters I and III or Annex XI Part A by 26 May 2028 (Extended deadline).
It’s important to note that by 26th September 2024 or earlier, manufacturers must sign a contract to continue selling in Europe after 2024. Contact us for more details.
Are you planning to apply MDR CE Certification for Reusable Surgical Instruments? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Technical Documentation for Class 1r Reusable Surgical Instruments
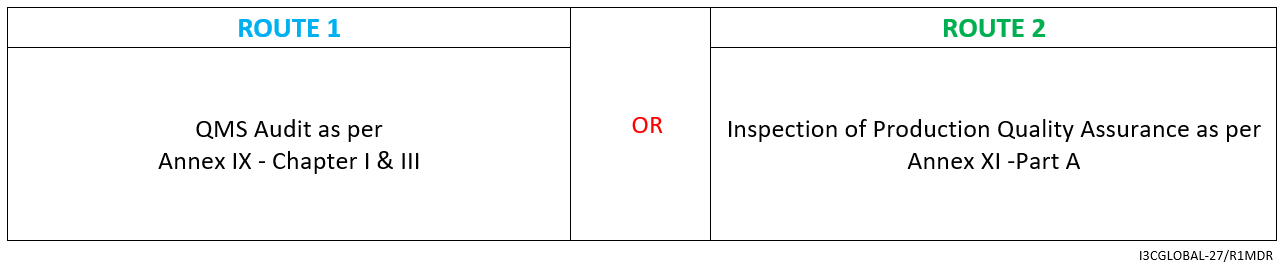
Below image explains the process and important documentation requirements for CE Certification for class 1r reusable surgical instruments.
CE Conformity Assessment Route
For Class 1 reusable surgical instruments conformity assessment route for CE certification as per MDR.
Do you need an email containing full details within 2 minutes?
Validation Studies for Class 1r Reusable Surgical Instruments
To showcase the efficacy of reusable device post usage do not affect the device’s acceptance criteria, so it is essential to conduct a cleaning validation. This validation should verify that both manual and mechanical cleaning methods allow for the effective reprocessing of the medical device. This information is crucial for manufactures to provide clear instructions to healthcare facilities via the user manual. Some of the key validation principles which organizations need to incorporate are:
- Robustness: It is about an examination which needs to be performed to check how device will perform in worst-case situation.
- Repeatability: The study aims to verify and confirm the variability of measurements. Repeatability should also validate the consistency of data and the process for specified outputs.
- Reproducibility: The study aims to examine potential variations within the system and ensure data integrity. Implementing multiple effectiveness cycles is necessary to account for variations in the test system. Reproducibility determines if the validation study can be repeated with consistent results.
Role of I3CGLOBAL Consultants in Class 1r CE Certification?
I3CGLOBAL Consultants play a significant role in assisting class 1r reusable surgical instruments manufacturers with the CE certification process. These devices, under the EU MDR, require In-depth documentation and records as reprocessing is involved before every use. We help manufacturers develop a regulatory strategy that aligns with the specific requirements under the MDR by identifying applicable regulations, guidance documents, and standards.
- We consultants prepare the technical documentation file ensuring it meets MDR requirements and submit to NB for review.
- Ensure Clinical Evaluation Report (CER) comply with MDR Article 61 and Meddev 2.7/1 rev 4 guidelines that includes clinical data, literature reviews, and post-market surveillance plans and reports
- We provide expertise in validating the reprocessing methods, ensuring that they are safe and effective for reuse.
- Guide the manufacturer through the necessary tests, such as cleaning, disinfection, sterilization, and functional testing.
- Liaison between the manufacturer and the Notified Body, helping to facilitate the technical file review process by responding to queries and providing additional documentation as required by the Notified Body.
I3CGLOBAL Consultants provide comprehensive support to class 1r reusable surgical instruments manufacturers ensuring complete compliance and documentation for CE Certification.
Are you planning to apply MDR CE Certification for Reusable Surgical Instruments? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Frequently Asked Questions
Is Class 1r considering a legacy device?
Reusable Surgical Instruments did not need the involvement of a notified body under MDD and have a Declaration of Conformity drawn up prior to 26 May 2021. Such manufactures as per Article 52(7) of the MDR, for the conformity assessment of class 1 reusable surgical instruments (Class 1r), the involvement of a notified body is necessary now. This sets these devices under the definition of a legacy device under Article 120(3) MDR.
All legacy reusable surgical device manufactures comply with the requirements of the extended transitional period, they can be placed on the market or put into service until 31 December 2028, provided they have a contract with Notified Body for the technical documentation assessment before 26th September 2024.
Why does a Class 1r reusable surgical instruments need a CE certification under MDR?
Under MDR, all reusable surgical instruments fall under the classification of class 1r must undergo Notified Body CE Certification before affixing CE mark.
What are the challenges associated with MDR CE certification?
Major challenges include ensuring compliance with sterilization validation, cleaning process validation and detailed documentation of reusability.
What are the costs associated with obtaining MDR Class 1r CE certification?
Class 1r CE Certification cost can vary widely depending on the Notified Body fees and testing fee and consultation fee
What is the difference between Class I and Class Ir medical devices under MDR?
Class 1 devices, typically low-risk and can be self-certified by the manufacturer. However, Class 1r devices, which are reusable surgical instruments, necessitate the involvement of a Notified Body specifically address the reusability aspects of the device.
