Quick Contact
Class 1m Medical Device
Class 1m medical device devices are intended for measuring body attributes, without surgical invasive procedures or connection with other devices. Class 1m measuring devices which measure physiological parameters or anatomical parameters or energy, respectively, or volume of medicinal products, body liquids or other substances administered to or removed from the body and display or indicate its value in a unit of measurement (example: urine bags, non-active thermometers, measuring spoons).
According to section 15.2 of Annex I, measurements made by devices with a measuring function will be expressed in legal units
Are you planning to apply MDR CE Certification for Class 1m medical device? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Class 1m Medical Device Technical Documentation
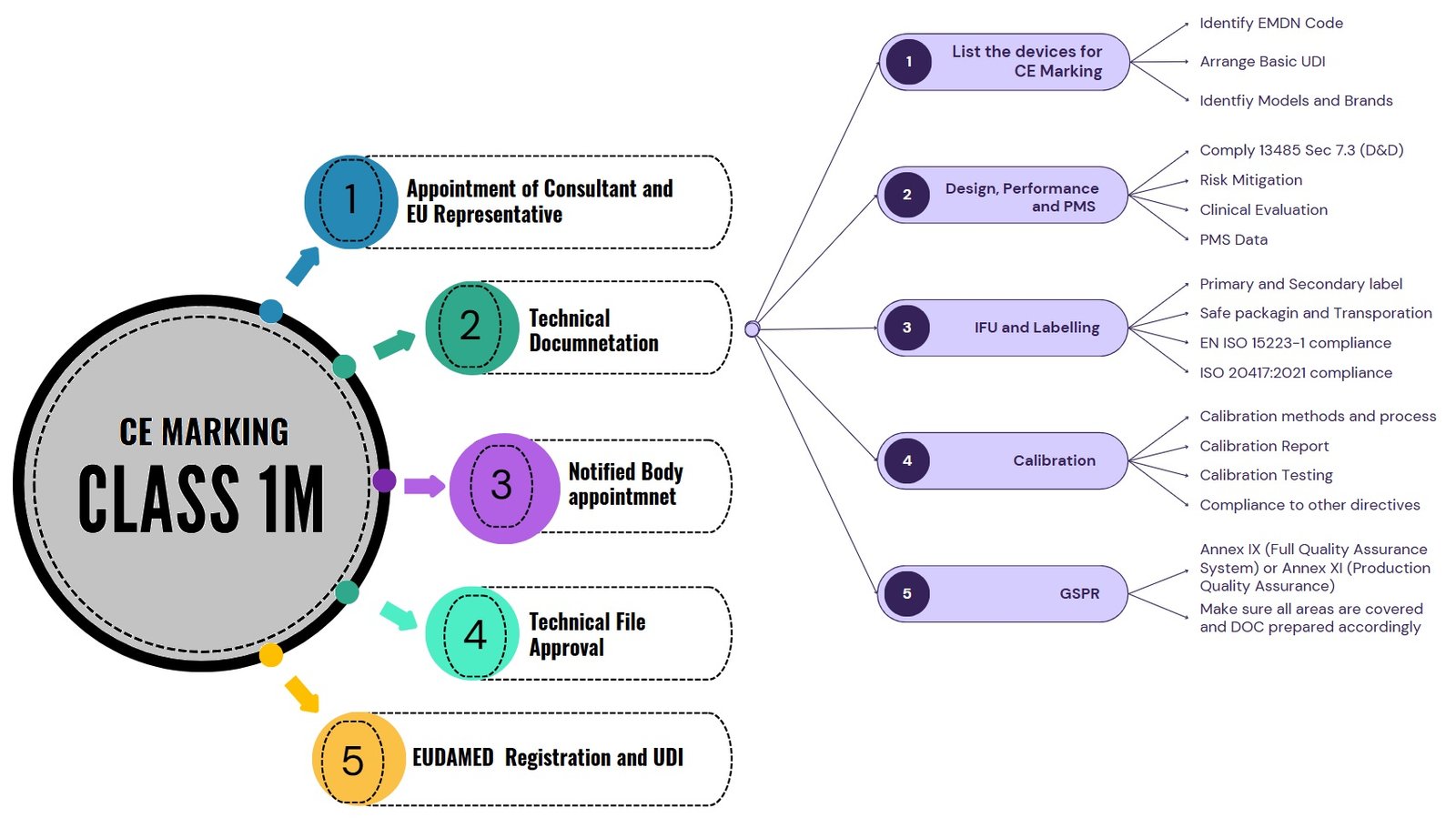
Technical documentation for a Class 1m measuring device is for demonstrating compliance with EU MDR 2017/745. Technical documentation includes detailed information about the device, such as its design, intended use, risk management, and performance characteristics.
Additionally, clinical evaluation documentation and post-market surveillance plans are integral components, ensuring that the device’s safety and performance are continually assessed throughout its lifecycle. For Class 1 measuring devices, a specific focus is placed on measurement accuracy, calibration procedures, and adherence to relevant harmonized standards. The below image explains the process and important documentation requirements for CE Marking for measuring device
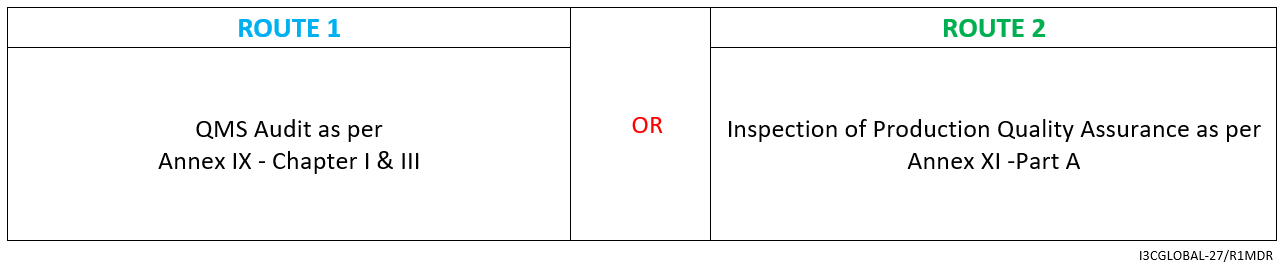
Class 1 Measuring Device CE Conformity Assessment Route
Class 1m medical devices require the intervention of a Notified Body. The Notified Body’s role is limited to assessing the aspects of the device related to its measuring function, ensuring accuracy and adherence to applicable harmonized standards or common specifications. For Class 1m measuring device conformity assessment route for CE certification as per MDR is mentioned below.
The Key MDR Requirements for Class 1m Medical Device
In the case of devices with a measuring function, to the aspects of manufacture concerned with the conformity of the devices with the metrological requirements.
- Accuracy refers to how close the measured value is to the true value.
- Precision refers to the device’s ability to consistently produce the same measurement under the same conditions.
These parameters must be validated and maintained records in the technical documentation file.
- Measuring devices often require regular calibration to ensure accuracy.
- Calibration involves comparing the device’s output against a known standard and making adjustments if necessary.
The measurement range specifies the limits within which the device can accurately measure. It should cover the full spectrum of expected measurements for the intended use of the device. The device must clearly indicate the units of measurement (e.g., mmHg for blood pressure, °C for temperature).
Do you need an email containing full details within 2 minutes?
Class 1m Medical Device Consultants
I3CGLOBAL Consultants play a significant role in assisting class 1 measuring device manufacturers with the CE certification process. These devices, under the EU MDR, require In depth documentation and records as reprocessing is involved before every use. We help manufacturers develop a regulatory strategy that aligns with the specific requirements under the MDR by identifying applicable regulations, guidance documents, and standards.
- We consultants prepare the technical documentation file ensuring it meets MDR requirements and submit to NB for review.
- Ensure Clinical Evaluation Report comply with MDR Article 61 and Meddev 2.7/1 rev 4 guidelines that includes clinical data, literature reviews, and post-market surveillance plans and reports
- We provide expertise in validating the reprocessing methods, ensuring that they are safe and effective for reuse.
- Liaison between the manufacturer and the Notified Body, helping to facilitate the technical file review process by responding to queries and providing additional documentation as required by the Notified Body.
- Guide the manufacturer through the necessary tests, such as cleaning, disinfection, sterilization, and functional testing.
I3CGLOBAL Consultants provide comprehensive support to class 1 measuring device manufacturers ensuring complete compliance and documentation for CE Certification.
Are you planning to apply MDR CE Certification for Class 1m device? We specialize in Technical Documentation, Clinical Evaluation, Risk Analysis and GSPR compliance ensuring manufactures achieve timely CE Certification.
Frequently Asked Questions
Why does a Class 1m medical device need a CE certification under MDR?
Under MDR, all non-sterile measuring device for measuring body attributes fall under the classification of class 1m must undergo Notified Body CE Certification before affixing CE mark.