Quick Contact
Find more about how we can help you with ISO 14971 Medical Device Risk Analysis

Biological Evaluation Report
Biological Evaluation Report documentation is crucial to Medical Device CE Marking, FDA 510k, UKCA Marking many other regulatory approval processes, and ongoing safety assessment. It involves assessing the device’s biological safety to ensure that it does not harm patients or users.
Biological evaluation typically follows international standards such as ISO 10993, which provides guidelines for evaluating the biological safety of medical devices.
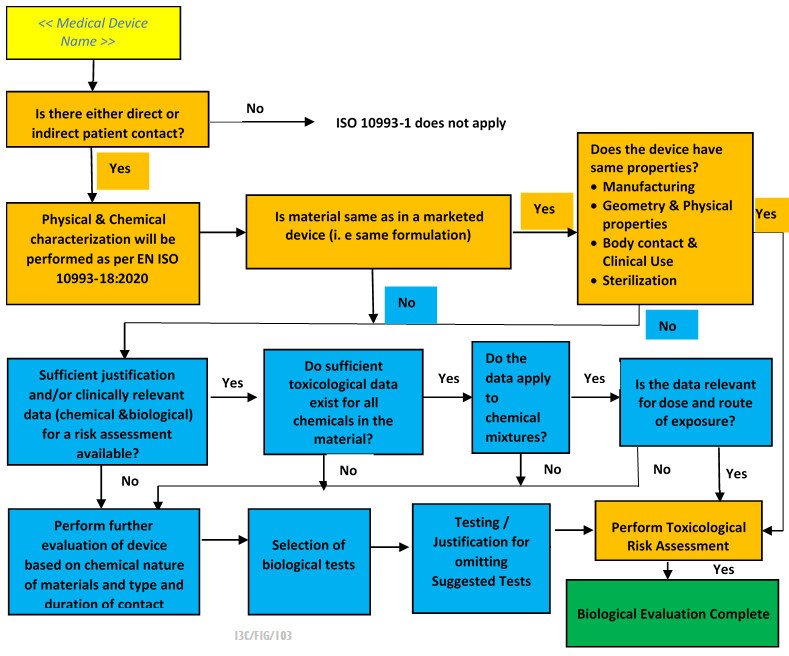
The Biological Evaluation Report Process normally includes the following steps:
- Identify potential biological hazards associated with the medical device.
- Evaluate the level of risk associated with each identified hazard.
- Conduct a series of tests to assess the biocompatibility testing of the medical devices.
- Characterize the chemical composition of materials used in the device and assess the potential for leachable substances to cause harm.
- Evaluation of clinical data on similar devices or materials, as well as any adverse events reported during clinical use.
- Implement risk management processes to mitigate any identified risks associated with the device.
- Document the results of the biological evaluation process in a report. This documentation is often submitted as part of MDR CE Marking or FDA 510k approval process.
Throughout the process, it’s essential to consider the specific characteristics of the device, the intended patient population, and the intended use environment

(Listen for 5 Minutes)
Importance of Biological Evaluation Report
Biological evaluation report is a collective summary of all biological tests performed and justifications for tests not performed. That includes supporting data from the literature, assessment of data, gap analysis for already available information for biological safety, rationale for why additional information isn’t needed, and a statement confirming the biological risk analysis and risk controls that have been completed.
The Biological evaluation report demonstrates that a medical device will not pose any potential risks to patients and intended users during its use.
The I3CGLOBAL and its regulatory professionals are qualified and experienced in taking on any risk class biocompatibility testing and biological evaluation report projects for MDR CE Marking and US FDA 510k submissions.
Biological Evaluation Plan
A Biological Evaluation Plan is a series of tests performed with the help of international standards preclinically, either through in-vitro or in-vivo techniques, and may utilize animal models to assess the biological safety of the medical device within the risk management process. The primary goal of the evaluation is to protect the patient from the biological risk raised by the medical device.
It is an important document that offers information on product safety as well as the technique used to evaluate device safety with available standards and guidelines. The biological Evaluation plan must demonstrate risk management activities in accordance with ISO 14971.
It is performed to measure the compatibility of a material with a biological system. The biological evaluation is performed using a series of tests as per a standard corresponding to the type of product for example pharmaceuticals, cosmetics, and medical devices to demonstrate that the material or device will not cause any potential risks to humans during its use.
A Biological Evaluation plan is used to prove the safety of the device by demonstrating compliance with corresponding standards and avoiding unnecessary testing of the device. Since evaluation is a risk management activity, a Plan is required, and this forms part of the Risk Management Plan.
The plan not only emphasizes the biocompatibility tests but also the requirements of ISO 14971 risk management. The plan should be drawn up by a knowledgeable and experienced team and that includes:
- arrangements for gathering of applicable information from the published literature
- arrangements for review and approval of the plan as part of the overall design control process
- arrangements for review of the final conclusions of the evaluation and the approval of any additional testing required
- arrangements for the final review and approval of the outcomes of the biological risk assessment
Biological Evaluation Report Template
Our team of experts and toxicologists after thorough interpretation of ISO 10993-1 latest version developed biological evaluation report template and necessary procedures for those looking for quick and easy solutions for developing supporting evidence for CE Marking or FDA 510k submission. The documents are in word format and easy to customize.
- Biological Evaluation Procedure : 150 USD
- Biological Evaluation Plan Template : 200 USD
- Biological Evaluation Report Template : 150 USD
High-quality biological evaluation report template and procedures for medical devices in compliance with EU & US FDA.
Biological Evaluation Report Factors
When conducting a biological evaluation, several essential factors need consideration to ensure a thorough and accurate assessment. Here are some key factors to consider:
- The material(s) of construction (i.e., all direct and indirect tissue contacting materials);
- Medical device configuration (e.g., size, geometry, surface properties)
- Intended additives, process contaminants and residues during the manufacturing of the device
- The manufacturing processes
- Packaging materials that directly or indirectly contact the medical device
- Leachable substances & Degradation products
- Other components and their interaction in the final product
- The performance and characteristics of the final product
- The physical and chemical characteristics of the device
- Systemic effects and local effects of implantable devices
- Biological hazards of every material and final product
- Existing toxicology and other biological safety data
The I3CGLOBAL and their professionals are qualified and experienced to take up any risk class Biocompatibility testing and biological evaluation report projects for MDR CE Marking and US FDA 510k submissions
Frequently Asked Questions
What is Surface-contacting devices?
- Skin: Medical devices that contact intact skin surfaces only, such as electrodes, external prostheses, fixation tapes, compression bandages, and monitors of various types
- Mucosal membranes: Medical devices that contact intact mucosal membranes like contact lenses, urinary catheters, intravaginal and intra-intestinal devices (stomach tubes, sigmoidoscopes, colonoscopes, gastroscopes) endotracheal tubes, bronchoscopes, dental prostheses, orthodontic devices, and intrauterine devices
- Breached or compromised surfaces: Medical devices that contact with breached or otherwise compromised body surfaces such as dressings, healing devices, and occlusive patches for ulcers, burns, and granulation tissue
What is External communicating devices?
These medical devices in contact with the following application sites are called external communicating devices
- Blood path, indirect: Medical devices or components that do not necessarily directly contact the blood path directly but serve as conduits to deliver fluids into the vascular system. Examples include solution administration sets, extension sets, transfer sets, and blood administration sets
- Tissue/bone/dentin: Medical devices that contact tissue, bone, or pulp/dentin systems such as laparoscopes, arthroscopes, draining systems, dental cement, dental filling materials, and skin staples. Medical devices or components that do not necessarily directly contact tissue or bone but serve as conduits to delivery to the tissue or bone, such as tubing used for irrigation
- Circulating blood: Medical devices that contact circulating blood like intravascular catheters, temporary pacemaker electrodes, oxygenators, extracorporeal oxygenator tubing and accessories, dialyzers, dialysis tubing and accessories, haem adsorbents, and immunosorbents.
What is Implant Devices?
These medical devices in contact with the following application sites are considered Implants
Tissue/bone:
- Medical devices principally contact bone such as orthopaedic pins, plates, replacement joints, bone prostheses, bone cement, and intraosseous devices.
- Medical devices principally contact tissue and tissue fluid such as pacemakers, drug supply devices, neuromuscular sensors, stimulators, replacement tendons, breast implants artificial larynxes, subperiosteal implants, and ligation clips.
Blood:
- Medical devices principally contact blood in the cardiovascular system such as pacemaker electrodes, artificial arteriovenous fistulae, heart valves, vascular grafts, internal drug-delivery catheters, and ventricular assist devices.
What are the applicable standards?
- EN ISO 10993-1:2020 – Biological Evaluation Report of Medical Devices – Part-1: Evaluation and testing within a risk management process
- Use of International Standard ISO 10993-1, “Biological evaluation – Part 1: Evaluation and testing within a risk management process”, September 4, 2020
- MDR 2017/745: Medical Device Regulation of the European Parliament and of the Council of 5 April 2017 on medical devices
Patient and User body contact area
Non-contacting medical devices are those which do not directly or indirectly contact the patient’s body. Diagnostic software, an in vitro diagnostic device and a blood-collection tube are examples of non-contact devices.
Surface-contacting medical devices are as follows;
Skin: Medical devices that contact intact skin surfaces only, such as electrodes, external prostheses, fixation tapes, compression bandages and monitors of various types.
Mucosal membranes: Medical devices that contact intact mucosal membranes like contact lenses, urinary catheters, intravaginal and intra-intestinal devices (stomach tubes, sigmoidoscopes, colonoscopes, gastroscopes) endotracheal tubes, bronchoscopes, dental prostheses, orthodontic devices and intrauterine devices.
Breached or compromised surfaces: Medical devices that contact with breached or otherwise compromised body surfaces such as dressings, healing devices and occlusive patches for ulcers, burns and granulation tissue.
Externally communicating medical devices: These include medical devices in contact with the following application sites,
Blood path, indirect: Medical devices or components that do not necessarily directly contact the blood path directly but serve as conduits to deliver fluids into the vascular system. Examples include solution administration sets, extension sets, transfer sets and blood administration sets.
Tissue/bone/dentin: Medical devices that contact tissue, bone or pulp/dentin systems such as laparoscopes, arthroscopes, draining systems, dental cement, dental filling materials, and skin staples. And, medical devices or components that do not necessarily directly contact tissue or bone but serve as conduits to delivery to the tissue or bone, such as tubing used for irrigation.
Circulating blood: Medical devices that contact circulating blood like intravascular catheters, temporary pacemaker electrodes, oxygenators, extracorporeal oxygenator tubing and accessories, dialyzers, dialysis tubing and accessories, haem adsorbents and immunosorbents.
Implant Medical Devices: These include medical devices in contact with the following application sites,
Tissue/bone:
Medical devices principally contact bone such as orthopaedic pins, plates, replacement joints, bone prostheses, bone cements, and intraosseous devices.
Medical devices principally contact tissue and tissue fluid such as pacemakers, drug supply devices, neuromuscular sensors, stimulators, replacement tendons, breast implants artificial larynxes, subperiosteal implants, and ligation clips.
Blood: Medical devices principally contacting blood in the cardiovascular system such as pacemaker electrodes, artificial arteriovenous fistulae, heart valves, vascular grafts, internal drug–delivery catheters and ventricular assist devices.
Patient and User body contact time
Contact duration categories: Medical devices shall be categorized as per the anticipated duration of contact as follows:
- Limited exposure (A): Devices whose single or multiple-use or contact is likely to be up to 24h
- Prolonged exposure (B): Devices whose single, multiple, or long-term use or contact is likely to exceed 24h but not 30 days.
- Permanent contact (C): Devices whose single, multiple, or long-term use or contact exceeds 30 days.
Transitory-contacting medical devices: Some medical devices with limited exposure have very brief/transitory contact with the body, such as lancets, hypodermic needles, and capillary tubes that are used for less than one minute, are excluded from biocompatibility testing. However, products made with materials such as coatings or lubricants that could be left in contact with body tissues after the medical device is removed will require a detailed biocompatibility assessment. Cumulative use should also be considered.
Medical devices with multiple contact duration categories: If a material or medical device can be placed in more than one duration category, a more rigorous evaluation will be considered. If a medical device, such as an absorbable glue, is intended to change during its lifetime, such as those that are polymerized and/or degraded in situ, the evaluation must consider all the different device states.


