Ancillary Medicinal Substance Dossier in MDR
Devices incorporating an ancillary medicinal substance, which are subject to Rule 14 of EU 2017/745 (MDR), must have their quality, safety, and usefulness verified in a manner consistent with the methods outlined in Annex I of Directive 2001/83/EC, also known as the Medicinal Products Directive
Annex IX, 5.2(a) of the MDR states the notified body should seek a scientific opinion from one of the competent authorities designated by the Member States by Directive 2001/83/EC or from the EMA, on the quality and safety of the substance including the benefit or risk of the incorporation of the substance into the device.
Annex II, Section 6.2 (a) states the documentation shall identify the source of that substance and contain the data of the tests conducted to assess its safety, quality and usefulness, taking into account the intended purpose of the device.
For their assessments, Medicines Competent Authorities (CAs) prefer documentation in the Common Technical Documentation (CTD) format. The CTD format is utilized for the pharmaceutical evaluation of medicinal products, and its use aids in streamlining the review process for CAs.
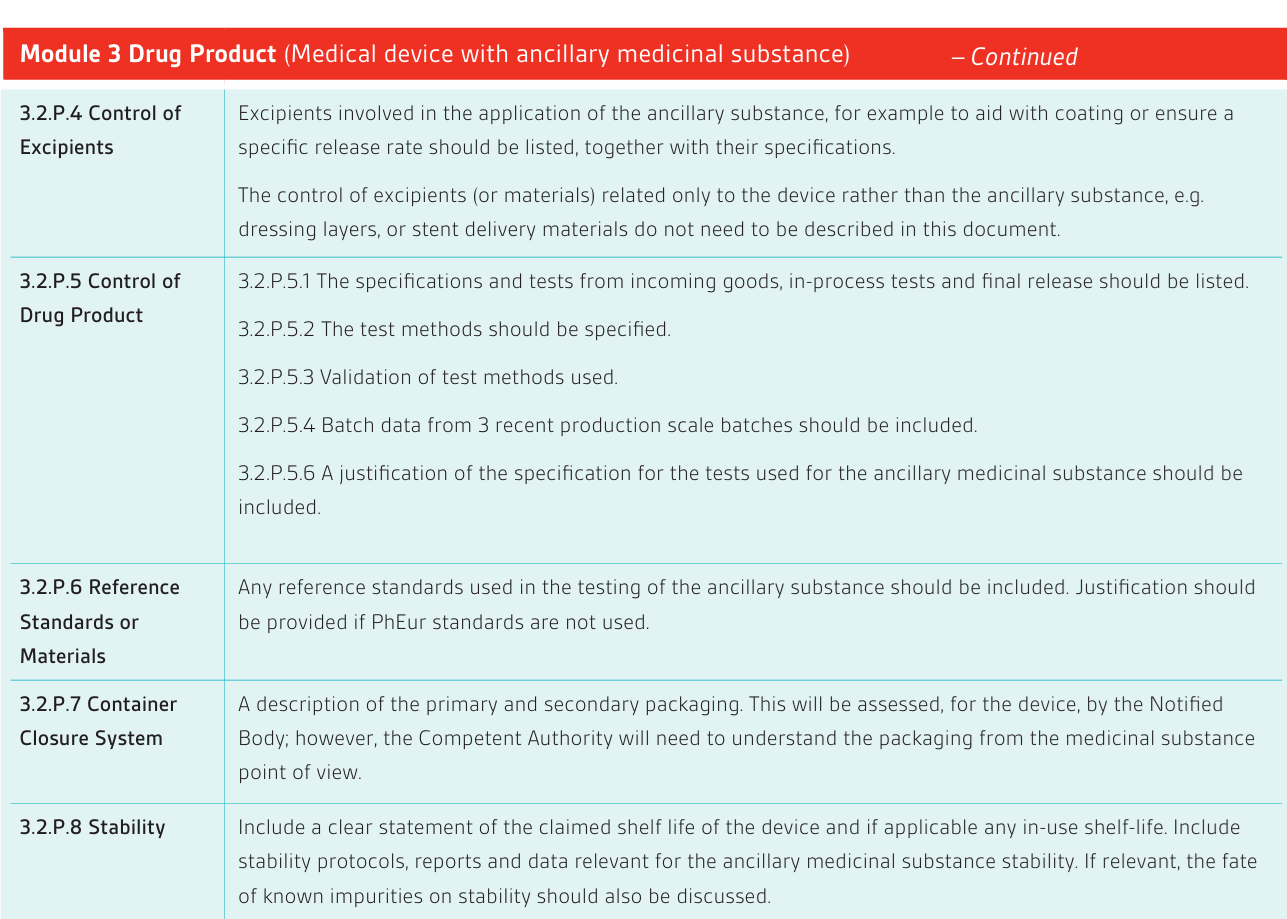
Dossier Requirements
In order to facilitate review, typically performed by a quality, a non-clinical and a clinical assessor; it is necessary to provide the dossier as separate, indexed and searchable (pdf) modules. Each module should be less than 100 MB to comply with Competent Authority requirements. If necessary supportive information can be provided in Appendices to the Modules. An index showing the folder structures and document titles should be provided. See reference section below for more detailed guidance, in particular the EMA dossier guidance.




**Image and information sourced from BSI