Quick Contact
MDR 2017/745
The new EU medical device regulation MDR 2017/745 supersedes the MDD 93/42/EEC, and the AIMDD 90/385/EEC. The new regulation was released in 2017, with its provisions becoming effective on May 26, 2022 (extended one year due to the COVID-19 pandemic). The transitional timeline for MDR will persist until December 31, 2028.
Are you planning for CE Marking? Do you have an MDR transition strategy in place? Don’t be concerned; you’re not alone. We are MDR 2017/745 consultants and regulatory experts.
New in MDR 2017/745
The MDR 2017/745 introduces additional requirements compared to MDD 93/42/EEC, without removing existing requirements. The following are the new requirements
- MDR 2017/745 widens the scope by including devices for cleaning, sterilization or disinfection of other medical devices, reprocessed single-use medical devices and certain devices without a medical purpose.
- MDR 2017/745 mandates a safety demonstration during the full product lifecycle, via clinical evaluation.
- The MDR 2017/745 places more stringent requirements on Notified Bodies.
- MDR 2017/745 mandates the consultation of an independent expert panel for the clinical evaluation of specific high-risk medical devices.
- The MDR 2017/745 introduces UDI – Unique Device Identifier.
- The MDR 2017/745 introduces the Eudamed database.
MDR 2017/745 Process Flow
Compliance with MDR 2017/745 requires adherence to a series of critical steps. Below is a summary of the process flow:
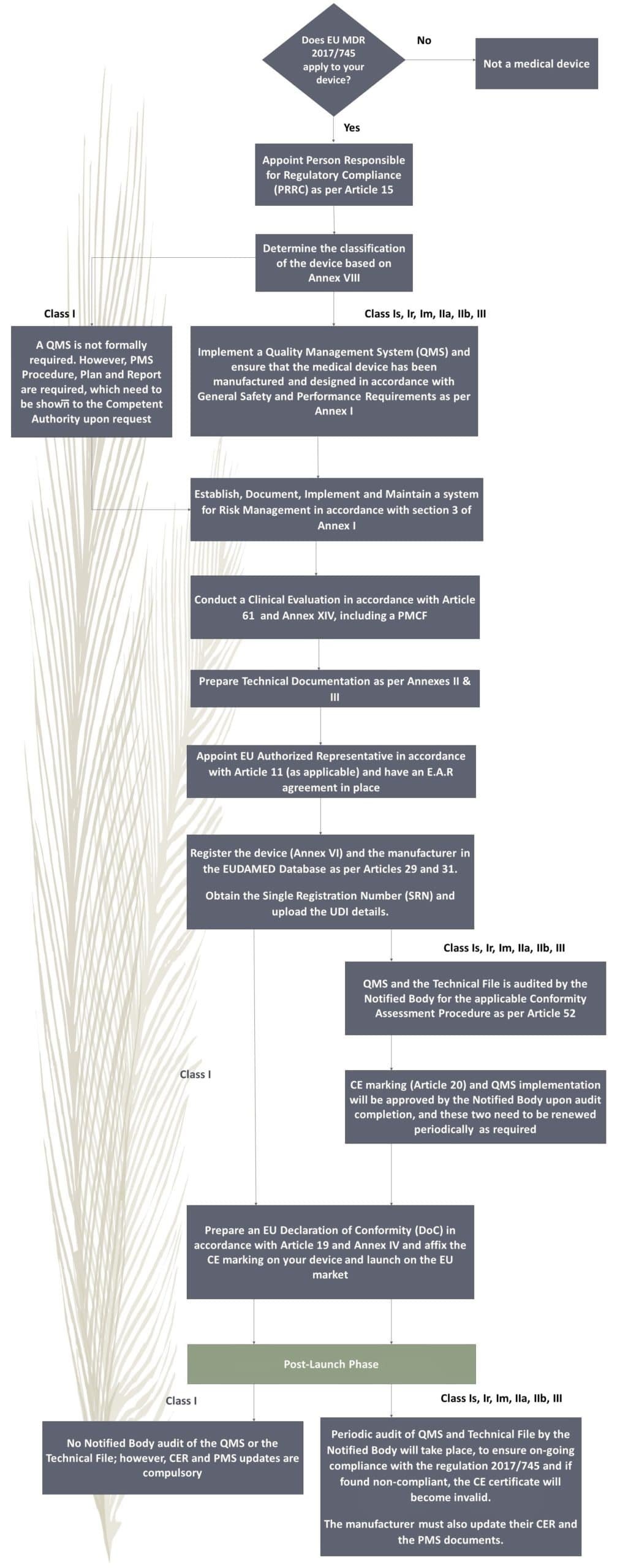
The cost of EU MDR 2017/745 consulting varies based type of medical devices, models, and variants within each device category. For more detailed information on how consulting costs are determined and to get a tailored quote for your specific needs, please fill out the quote request form.
Frequently Asked Questions
Is there any guide for standardization for medical devices?
Which device types are covered in MDR 2017/745?
The Medical Device Regulation encompasses all medical devices formerly under the Medical Device Directive (MDD, 93/42/EEC) and products governed by the prior Active Implantable Medical Device Directive (AIMDD, 90/385/EEC).



