UKCA Marking For Medical Devices!
We possess complete technical and scientific expertise, along with highly qualified resources, to achieve UKCA marking for medical devices and in vitro diagnostic devices placed on the market in Great Britain, including England, Wales, and Scotland
Contact Us
What is UKCA Marking?
UKCA Marking is a valid indicator that a medical devices conform to relevant UK Regulations. The UKCA certification is mandatory for medical devices sold in Great Britain (England, Wales, and Scotland) post-Brexit. The UKCA Mark is the UK equivalent of the EU CE Mark.
The United Kingdom’s Medicines and Health Care Products Regulatory Agency (MHRA) published new guidelines to regulate medical devices after Brexit. The UKCA will not be recognized in the EU, EEA, or Northern Ireland, and products still require CE Certification for sale in these markets. The manufacturer or their authorized representative will be responsible for affixing the UKCA certification to the product, following the same principle as for CE marking but for the UK market.
A third-party evaluation procedure by a UK-approved body is required for UKCA Marking sold in the UK. This procedure is comparable to that used in the European Union for CE Certification, in which the EU Authorized Notified Body assesses compliance. The MHRA has stated that the UKCA Marking will be based on the IVD, MDD, and AIMD Directives until June 30, 2023, rather than the EU MDR 745/2017 and EU IVDR 746/2017.
Importance of UKCA Marking
UKCA marking is the UK’s mandatory certification for medical devices and in vitro diagnostic (IVD) devices, ensuring they meet the regulatory standards set by the Medicines and Healthcare Regulatory Agency (MHRA). Following Brexit, UKCA marking has replaced the CE marking after Brexit.
Compliance with the UK Medical Devices Regulations 2002, as amended, is essential for manufacturers to legally market their products. UKCA marking signifies that a device is safe, effective, and meets stringent quality standards, safeguarding public health and ensuring regulatory compliance.
UKCA Marking Consultants
UKCA Marking consultants play a crucial role in assisting manufacturers in ensuring compliance with UK regulations (MDR 2002) post-Brexit. Here are the important technical areas.
UKCA (UK Conformity Assessment) is the new UK product marking required for medical and In-vitro diagnostic devices sold in Great Britain (England, Scotland, and Wales) after Brexit. It replaces the CE marking that was previously used under EU regulations. I3CGlobal Consultants helps manufacturers navigate the complex regulatory landscape and ensure their devices meet MHRA Certification Body UKCA marking compliance requirements.
- Consultants specializing in UKCA marking for medical devices and IVDs possess extensive knowledge and experience in regulatory requirements and standards specific to the UK market. They stay updated on any changes in regulations and guidelines, providing manufacturers with accurate and timely advice.
- Ensuring compliance with technical documentation to demonstrate the safety and efficacy of medical devices and IVDs. Consultants help manufacturers identify the documentation needs, risk analysis, and clinical evaluation, address compliance issues, and implement appropriate strategies.
- Navigating regulatory requirements can be time-consuming and challenging for manufacturers, especially those unfamiliar with the UK regulatory framework. Consultants streamline the process, helping manufacturers save time and resources by guiding the most efficient pathways to UKCA marking.
- UKCA Marking and certification consultants assist manufacturers in preparing the necessary documentation, supporting evidence and technical files required for UKCA marking, including DOC, clinical, risk assessments, and quality management system documentation. They ensure that all documentation meets the requirements of UK regulations.
- Consultants provide support during Onsite audits by UKCA Certification Bodies, helping manufacturers demonstrate compliance with UKCA marking requirements and addressing any issues that may arise during the audit process.
The I3CGLOBAL team guide you through the medical and IVD rules, covering all device classes and types. We assist in handling further comments from UK CB or MHRA and communicate effectively and comprehensively. All of these tasks require exceptional regulatory, technological, and scientific expertise, as well as adequate manpower.
Our UKCA Marking Consultant possess the necessary experience and credentials to serve as regulatory consultants and UKRPs. It is not advisable to appoint your UK Importer as the UKRP, as this helps segregate your business and regulatory interests in the United Kingdom.
UKCA Marking Technical File
Members of the QA and regulatory teams frequently struggle to discover the requisite number of medical device technical files for devices slated for UKCA Marking. The correct segmentation of technical files based on intended usage, application, and GMDN Code is critical for the file’s quick processing.
It is advised to remember that the medical device technical file or documentation is all about your device to establish safety and performance. It means you cannot combine products with different intended uses, different classes, different construction materials, or even different designs.
- Device Intended use and Indication of use
- Site of application
- Design change or major constructional changes
- State of the art
- Addition of multiple directives or regulations
- Evaluation of accessories, components, and software/firmware
UKCA Conformity Assessment Bodies
The UK Notified Body accreditations will be revoked on December 31, 2020, and their CE marking will no longer be acceptable for placing medical devices and in vitro diagnostic equipment on the UK market. After December 31, 2020, UK-based Notified Bodies will automatically become UK Conformity Assessment Bodies, and their CE Certificates will no longer be valid.
The common question raised by EU manufacturers is whether the CE Mark Certificate issued by an EU Notified Body will be accepted by a UKCA UKCA-approved body and utilized to produce a UKCA Certification for the same medical device. The response is that if EU Notified Bodies are willing to share information with UKABs when the certificate holder asks for it, UKABs will be able to provide UKCA Certification without having to repeat the entire certification process. Cooperation between EUNB and UKAB is vital; thus, we assess the possibilities on an individual basis and guide manufacturers accordingly.
It’s important to remember that conformity assessment entails an audit of the manufacturer’s quality management system as well as a review of technical documentation related to the products to be CE Marked for specific criteria. As a result, if your EU-notified body isn’t cooperating with a UKAB, additional on-site audits and technical documentation reviews will be required, and manufacturers will have to pay a hefty fee for the process.
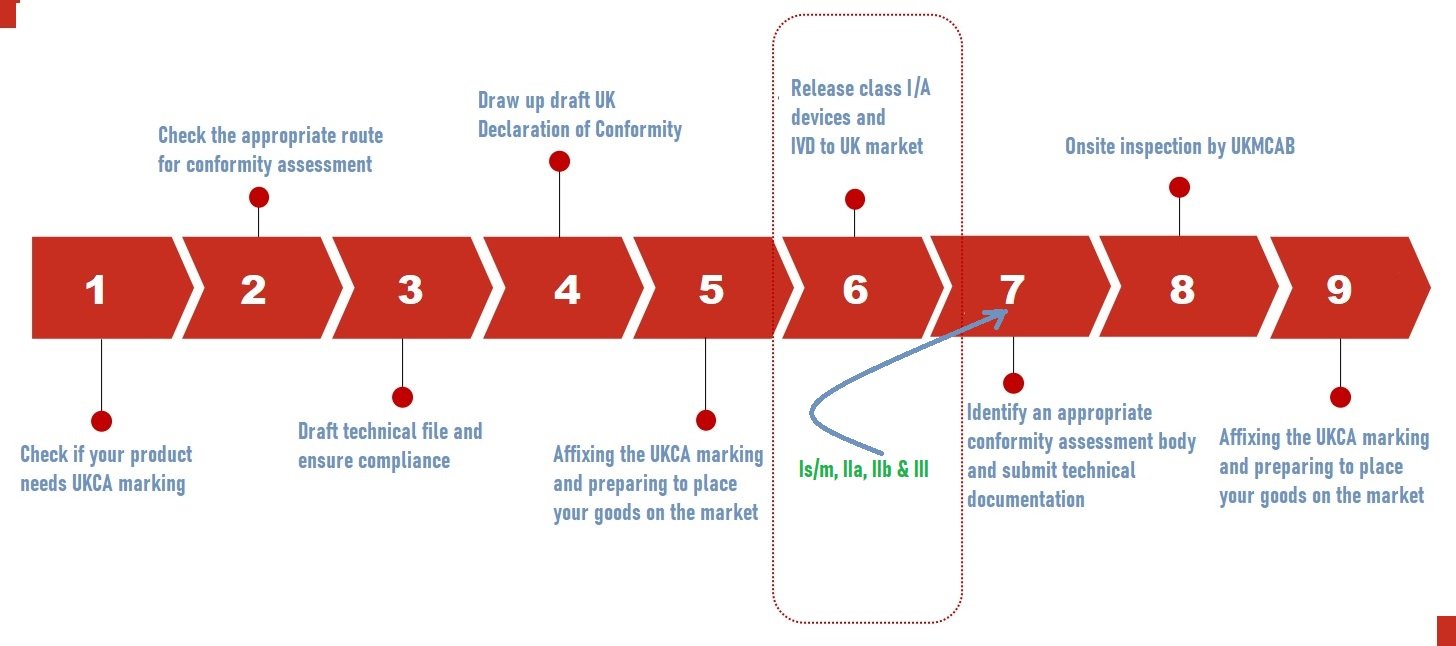
UKCA Certification Process
The conformity assessment for the UKCA Certification process starts with the understanding and interpretation of UKMDR’s latest regulation, selecting suitable conformity assessment methods, performing required testing, compiling technical documentation, and possibly engaging a UK Approved Body. This process assures that products conform to UK standards regarding safety, health, and environmental protection.
- Identify the directives and standards that apply to you.
- Double-check the prerequisites.
- If relevant, conduct pre-clinical, safety, and performance investigations.
- Prepare a technical file and a compliance statement.
- The technical material should be submitted to the Conformity Assessment Body (UK CAB).
- The onsite inspection was followed by a review of the technical files.
As per UK MHRA class 1 devices and Class A devices can be self-certified followed by MHRA registration to sell in the UK. This is a comparable easy process and does not involve UKCA bodies. Refer to the below image for the simplified UKCA process flow chart.
I3CGLOBAL was established in 1999 in the CE Marking regulatory consulting service industry. We have 100+ full-time experienced regulatory professionals in the CE marking process which is very similar to UKCA Marking. Our team has extensive knowledge of any risk class of device.
Frequently Asked Questions
What is average timeline for UKCA marking?
The average timeline is around 8 months post-submission of the technical documentation.
What is average timeline for preparing the technical documentation?
With the full support of the manufacturer, the consultant team may be able to finish the technical documentation in 3-4 months. In some cases, the implant timeline may extent to another 2 more months.
What is average timeline for completing Biocompatibility testing?
The majority of the tests can be completed in 90 days.
Will the I3CGLOABL team Authorize / Prepare technical file for the clients?
Yes. We prepare the technical documentation for all our customers with assurance. We also submit and answer CB queries.