What is Infusion Set?
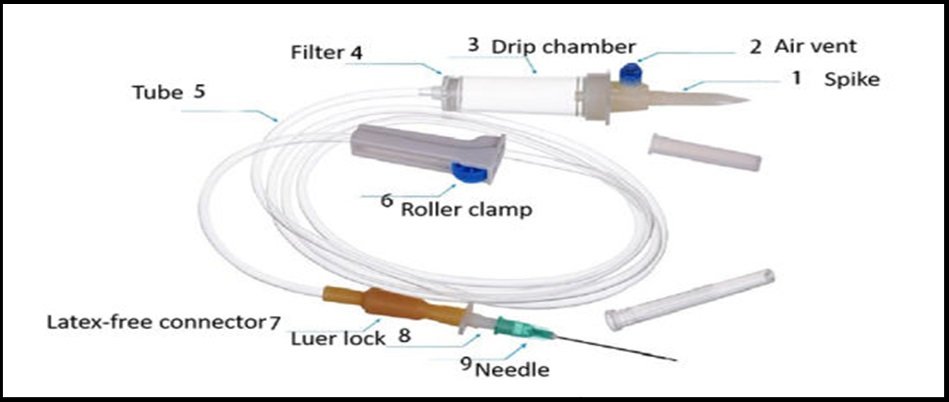
Infusion sets are medical devices used in healthcare settings and for at-home medical treatments to deliver fluids, medications, or nutrients directly into a patient’s circulatory system or subcutaneous tissue. These sets typically consist of various components designed to administer fluids or medications safely and effectively.
Components:
- An intravascular administration set is a tool used to inject a needle or catheter into a vein to deliver fluids from a container to a patient’s vascular system.
- The device may consist of the following: a side tube with a cap to serve as an injection site, tubing, a flow regulator, a drip chamber, an infusion line filter, an I.V. set stopcock, fluid delivery tubing, connectors between parts of the set, and a hollow spike to penetrate and connect the tubing to an I.V. bag or other infusion fluid container.
Types of Infusion Sets that need FDA 510K Clearance
- Intravenous (IV) Infusion Sets: These deliver fluids directly into a vein and are commonly used in hospitals for fluid resuscitation, medication administration, or blood product transfusions.
- Subcutaneous Infusion Sets: These are used for continuous or intermittent infusion of medications or fluids under the skin. They are often used for insulin delivery in diabetic patients.
Purpose / Intended Use of Infusion Sets
Intravascular administration sets are versatile and can be used with various methods of fluid delivery, such as gravity flow (passive flow based on height difference), pump-assisted flow (using a mechanical pump), subcutaneous infusion (under the skin), or implanted ports. The specific components and features of the set can vary based on the intended use and patient needs, ensuring the safe and effective administration of fluids and medications.
Material Types and Additional Features:
IV sets may include Diethylhexylphthalate (DEHP) and non-DEHP PVC tubing, co-extruded tubing, calibrated burettes, filters, standard and needle-free Y-injection sites (swabbable and non- swappable), anti-siphon valves, and check valves to prevent retrograde fluid flow.
FDA Code for Different Types of Infusion Sets
| Type | Product code | Class |
| Set, blood transfusion | BRZ | 2 |
| Stopcock i.v set | FMG | 2 |
| Set, administration, intervascular | FPA | 2 |
| Filter, infusion line | FPB | 2 |
| Tubing fluid delivery | FPK | 2 |
| Set, i.v fluid transfer | LHI | 2 |
| Check valve, retrograde flow (in-line) | MJF | 2 |
| Microfilter, Blood Transfusion | CAK | 2 |
| System/Device, Pharmacy Compounding | NEP | 2 |
| Administration Set Docking Station | ODI | 2 |
| Intravenous Extension Tubing Set | OJA | 2 |
510K Documentation Requirements for Infusion Sets
Read More information about 510k mandatory documentation
Biocompatibility Test requirements for Infusion Sets
We recommend that you conduct biocompatibility testing for your device as described in the guidance entitled, Use of International Standard ISO-10993, Biological Evaluation of Medical Devices Part 1: Evaluation and Testing.
- Cytotoxicity -ISO 10993-5
- Sensitization- ISO 10993-10
- Intracutaneous- ISO 10993-10
- Systemic toxicity- ISO 10993-11
- Acute systemic toxicity- ISO 10993-11
- Hemolysis- ASTM F756
- Complement activation -ISO 10993-4
- Thrombosis -ISO 10993-4
- Pyrogen -USP <151>
Performance Test requirements for Infusion Sets
We recommend that you conduct all testing based on the device‘s intended use.
- EN ISO 8536-8:2015 Infusion equipment for medical use – Part 4: Infusion sets for single use, gravity feed.
- ISO 8536-9:2015 Infusion Equipment for Medical Use – Part 9: Fluid Lines for Single Use with Pressure Infusion Equipment SO 11737-1:2018, Sterilization of medical devices – Microbiological methods – Part 1: Determination of a population of microorganisms on products.
- ISO 11737-2:2019, Sterilization of medical devices – Microbiological methods – Part 2: Test of sterility performed in the definition, validation, and maintenance of a sterilization process.
- Needle Performance – ISO 7864 and ISO 9626
- Functional tests
- Leak/Tightness
- Flow (Occlusion)
- Tensile tests
- Microbial Ingress Testing
- ISO 80369-7:2016 Small-bore connectors for liquids and gases in healthcare applications-Part 7: Connectors for intravascular or hypodermic applications.
Sterilization and Shelf-life requirements for Infusion Set
- Method (ETO/Gamma)
- Validation Method
- Dosage (if gamma irradiated)
- Residues (if ethylene oxide)
- Sterile Barrier Packaging testing
- Seal strength ASTM F88/F88M-15
- Dye penetration test- ASTM F1929 -12
- A shelf life of 3 years is validated using the FDA-recognized standard ASTM F1980-16 Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices.